Q1. What is Clinical evaluation? Karnataka
Ans. Clinical evaluation is a methodologically sound procedure to collect, appraise
and analyse clinical data related to a medical device and to assess whether there is
sufficient clinical evidence to confirm compliance with the general safety and performance requirements of the device when it is used as intended by the manufacturer. it is done, initially to obtain CE marking of the device and for placing the device in the market but updated thereafter periodically as new information becomes available from ongoing research works or literatures published.
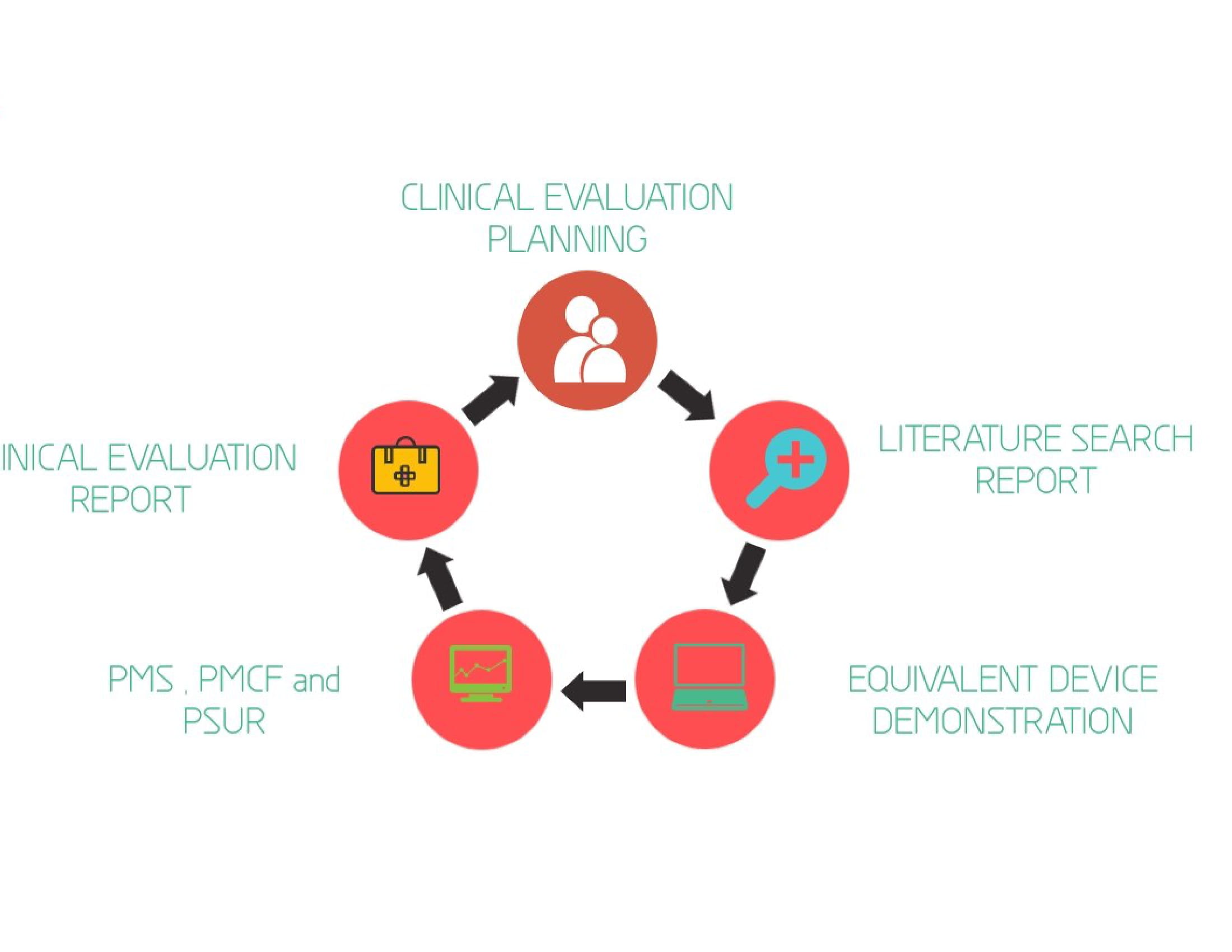
The clinical evaluation is based on a comprehensive analysis of available pre- and post-market clinical data relevant to the intended purpose of the device. There are 4 different stages in performing clinical evaluation:
- Stage 0: Scoping and planning of clinical evaluation
- Stage 1:Identification of pertinent data (identifying relevant pre-market and post-market clinical data)
- Stage 2: Appraisal of pertinent data (for scientific validity and relevance)
- Stage 3: Analysis of the clinical data (in order to reach to conclusions about General safety and performance requirements)
- Stage 4: Finalise the clinical evaluation report ( The report should summarize the data analysis).
The Clinical Evaluation report should provide strong clinical evidence for conformity assessment so that the device can be approved for sale in European markets.
Q2. What is the relevance of the clinical evaluation in the Technical documentation mentioned in the MDR?
Ans. Every medical device sold in Europe, whether new or existing, must have an updated clinical evaluation report as part of its Technical file. As per Annex II of MDR, the clinical evaluation report and its updates are part of technical documentation of device and the manufacturers shall plan, conduct and document clinical evaluation in accordance with Article 61 and Annex XIV of MDR. Clinical Evaluation Report
As per Article 61, clinical evaluation shall follow a defined and methodologically sound procedure based on:
- Evaluation of the relevant scientific literature currently available relating to the safety, performance, and intended purpose of the device, if the device is equivalent to an already marketed device
- Critical evaluation of the results of all available clinical investigations
- a consideration of currently available alternative treatment options, if any.
The results of the clinical evaluation and the clinical evidence on which it is based shall be documented in a clinical evaluation report which shall support the assessment of the conformity of the device. Both favourable and unfavourable data considered in the clinical evaluation shall be included in the technical documentation.
The clinical evaluation report shall be updated throughout the life cycle of the device with clinical data obtained from Post market surveillance, post market clinical follow-up reports.
Q3. Is it mandatory to conduct the clinical evaluation for all classes of the medical devices as per MDR?
Ans. Yes. Clinical Evaluation is mandatory for all classes of Medical devices as per MDR. It should be appropriate in view of the characteristics of the device and its intended purpose.
Q4. I am a manufacturer of a class III implantable device, which is integrated by medicinal substance. Is it possible to conduct the clinical evaluation with the exception from the clinical investigation?
Ans. For class III devices and implantable medical devices, the clinical investigation need not be performed if the following conditions are met,
- the device is designed by modifications of a device already marketed by the same manufacturer
- the modified device is equivalent to the marketed device, and demonstration of equivalence has been endorsed by the notified body, and
- the clinical evaluation of the marketed device is sufficient to demonstrate conformity of the modified device with the relevant safety and performance requirements.
In case if the device is equivalent to an already marketed device not manufactured by the same manufacturer, Clinical investigation need not be performed,
- If the two manufacturers have a contract, which allows the manufacturer of the second device full access to the technical documentation of the first device, and
- the original clinical evaluation has been performed in compliance with the requirements of Regulation.
Q5. How frequently we have to update the clinical evaluation report?
Ans.
The clinical evaluation report should be updated throughout the life cycle of the device with clinical data obtained from Post market surveillance and Post market clinical follow up reports. The following things should be considered while determining the frequency of updates:
- Whether the device carries any significant risks
- Whether it is well established
- Whether there are uncertainties and unanswered questions about the device
- Change in the design or manufacturing procedures if any
The clinical evaluation should be actively updated, when some new data is received from post market surveillance reports which can alter the current clinical evaluation. Declaration of Conformity
Otherwise, it should be updated:
- Annually, if the device is a high-risk device or if it is new to market
- once in a 2 to 5 years, if the device is a low risk device or if it is already established.
Q6. What are considered as Clinical data asper MDR?
Ans. Clinical data is all information related to safety and performance of device that is generated by using the device. This could be data from
- clinical investigations of the device under evaluation
- clinical investigation or other studies reported in the scientific literature, of a similar device for which equivalence to the device in question can be demonstrated
- published and/or unpublished reports on other clinical experience of either the device in question or a similar device for which equivalence to the device in question can be demonstrated.
Q7. When the literatures are selected for the clinical data what all have to be considered?
Ans. The literature collected may relate directly to the device in question (e.g. publications of clinical investigations of the device in question that have been performed by third parties, its side effects or complications, incidence reports) and/or to equivalent device, benchmark devices, other devices and medical alternatives available to the intended patient population.
The selection of literature should be objective and justified, i.e. include all relevant data, both favourable and unfavourable. It is important that the literature search is documented to such degree that the methods can be appraised critically, the results can be verified, and the search reproduced if necessary.
Q8. What are the aspects of the state of the art to be covered for the literature search?
Ans. The following aspects should be covered. The current knowledge in the corresponding medical field, such as
- applicable standards and guidance documents,
- information related to the medical condition managed with the device and its natural course and consequences,
- information related to benchmark devices, other devices and
- alternative methods available to treat the disease